Category: Industry Information
Date: 2018-07-11
Click: 5595
Author: admin
Collection:
After 47 minutes, the vacuum-injected battery is basically infiltrated, but the lithium-ion battery in the atmospheric pressure injection still has a considerable part of the intermediate position.
Lithium Grid News: Xiao Bian has summed up a word that is crucial for lithium-ion batteries in his years of work experience - "even". Why is this word so important? From the perspective of the entire production process of lithium batteries , the first is the homogenization process. The purpose of homogenization is to mix the components of the active material, conductive agent and binder “evenly” (marked); followed by coating. The key to the process is to ensure the "uniformity" of the coating amount, to avoid problems such as poor product consistency caused by the fluctuation of the coating amount; then, the liquid injection process, and also to ensure the "uniform" infiltration of the electrolyte inside the cell to ensure the battery Performance and cycle life; the most important thing in the production process of battery modules is the uniformity of the single cells, that is, "uniformity" to ensure the full performance of the battery pack.
How to ensure the electrolyte in lithium within the cell batteries fully, evenly infiltration is a troubled years of lithium production problem, numerous lithium engineers paid a lot of effort, Bosch engineers WJ Weydanz et al. [1] neutron Diffraction technology successfully observed the infiltration process of the electrolyte inside the soft-packed lithium-ion battery (as shown in the figure below, a, b, c vacuum injection, d, e, f atmospheric injection), you can see the injection for 2min Most of the electrolyte still stays outside the cell. After 47 minutes, the vacuum-injected battery is basically infiltrated, but the lithium-ion battery in the atmospheric pressure injection still has a considerable part of the intermediate position. The cell is not infiltrated. A large amount of electrolyte remains on the outside. WJ Weydanz's research shows that vacuum injection can reduce the injection time of lithium-ion batteries by 50% and increase the injection rate by 10%, which is of great significance for improving the quality and efficiency of injection.
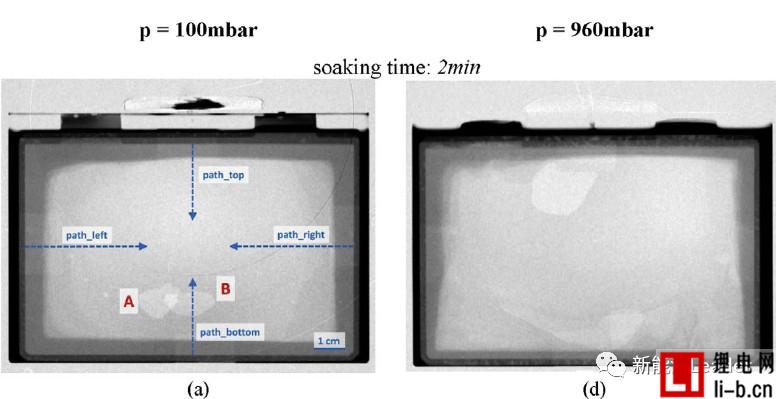
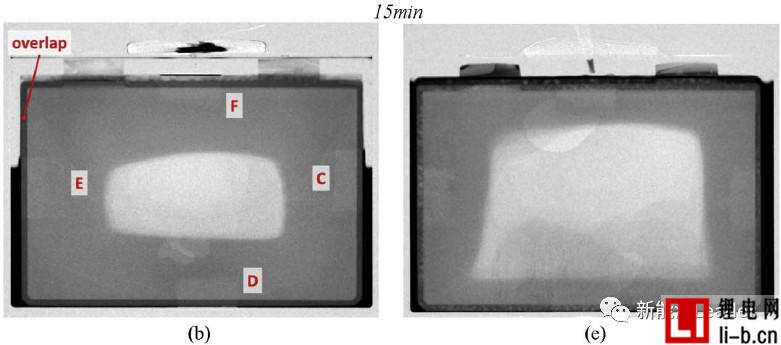
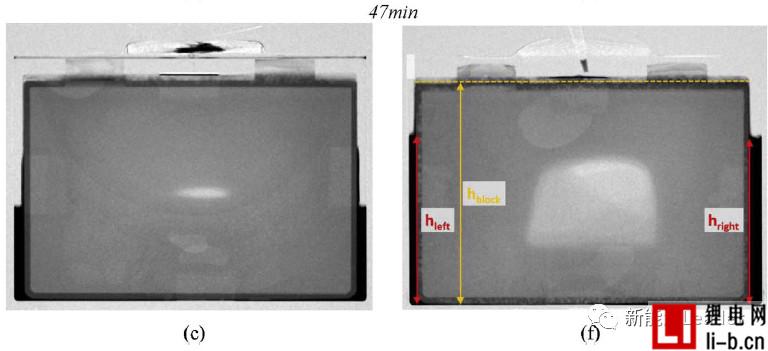
Generally speaking, gravity has a certain influence on the wettability of lithium ion batteries. Therefore, in order to ensure the infiltration effect, it is necessary to periodically “turn over” the lithium ion battery after injection, but WJ Weydanz analyzes the upper and lower directions. The infiltration speed, it is found that the influence of gravity on the infiltration process of the electrolyte is minimal, and the influence of gravity on the infiltration of the lithium ion battery can be basically ignored.
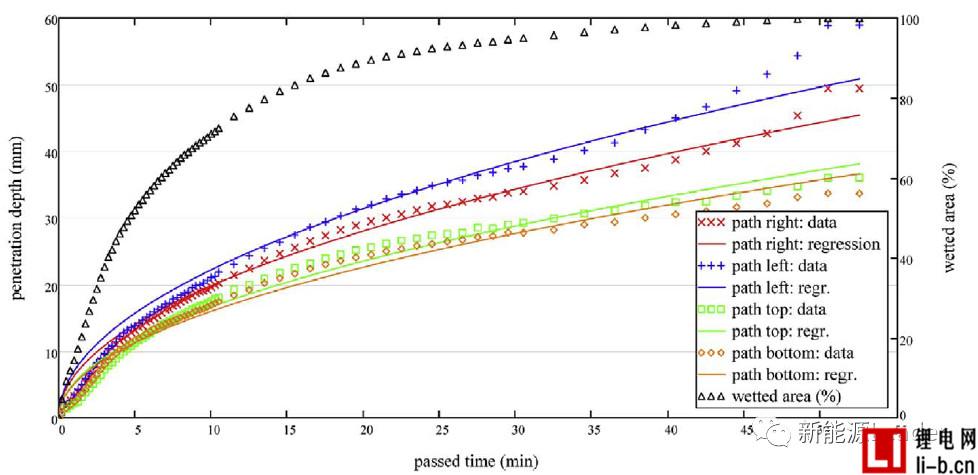
Vacuum injection can improve the wettability of lithium-ion batteries and increase the amount of liquid injection. It has become the consensus in the industry, but what kind of vacuum injection system can maximize the effect of vacuum injection? To this end, Thomas Knoche et al. [2] of the Technical University of Munich analyzed the effect of the vacuum system on the effect of liquid injection. The main difference between the two vacuum processes below is the degree of vacuum and the timing of sealing.
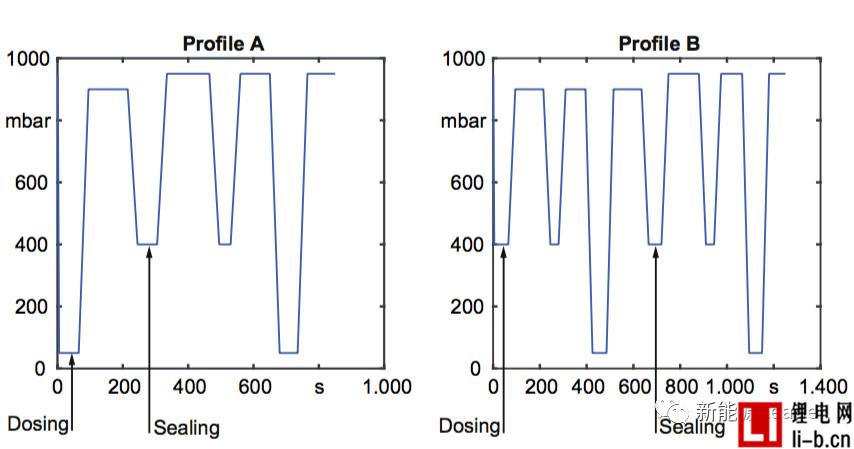
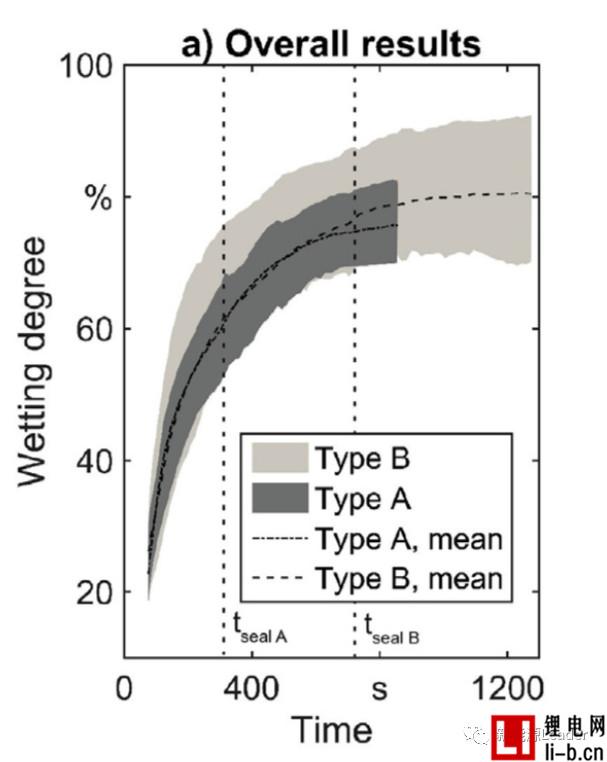
Thomas Knoche obtained the infiltration rate data by analyzing the proportion of the infiltrated area. The following figure a shows the relationship between the infiltration condition and the time after the liquid injection in the two vacuum systems. From Fig. a, it can be seen that the number of vacuuming after the injection is more In the B system, the average infiltration rate was 78.73% at 850s, and the average infiltration rate of the 850s in the A system with less vacuum after injection was 73.18%, which indicates that several vacuums after injection are beneficial to increase electrolysis. The infiltration effect of the liquid.
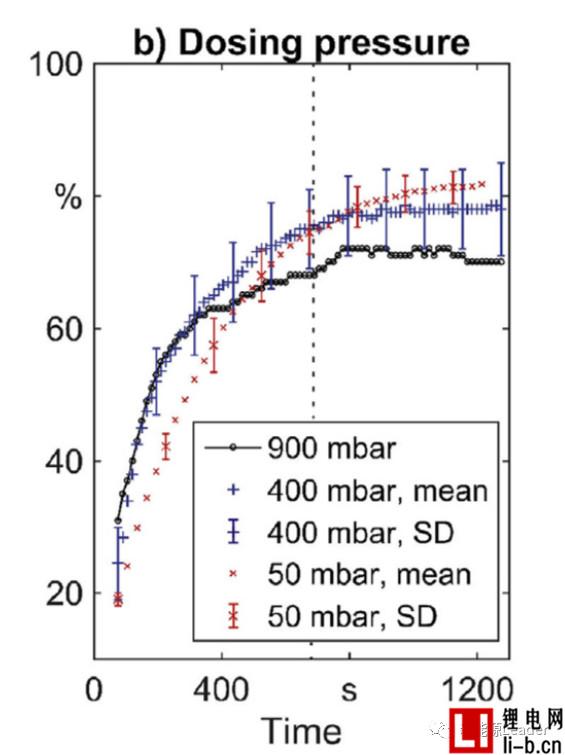
Figure b below shows the relationship between cell infiltration rate and time after liquid injection under different vacuum degrees. The final infiltration rates of the cells after injection at 50 mbar, 400 mbar and 900 mbar are 82.3%, 77.9% and 70.1%, respectively. The liquid vacuum has a significant effect on the wetting effect. The higher the degree of vacuum at the time of liquid injection, the better the effect of the final electrolyte on the cell.
Here are some benefits for everyone. The following video is the whole process of electrolyte infiltration observed by Thomas Knoche through neutron diffraction technology. This is also the first time that Xiaobian saw the infiltration process of electrolyte in the cell. I hope to help you lithium battery engineers.
Friends who don't see enough video traffic can quickly learn about this animation:

In addition to the improvement of the liquid injection process, the choice of the diaphragm also has a significant effect on improving the electrolyte wetting effect. The common lithium ion battery separators are mostly PE, PP single layer or multilayer composite structure diaphragms, which have very good diaphragms. Stability, therefore widely used, but this non-polar polymer membrane is incompatible with polar cyclic carbonate solvents (such as EC, PC), resulting in wettability between the electrolyte and the membrane Very poor, it also directly affects the electrolyte infiltration effect on the core.
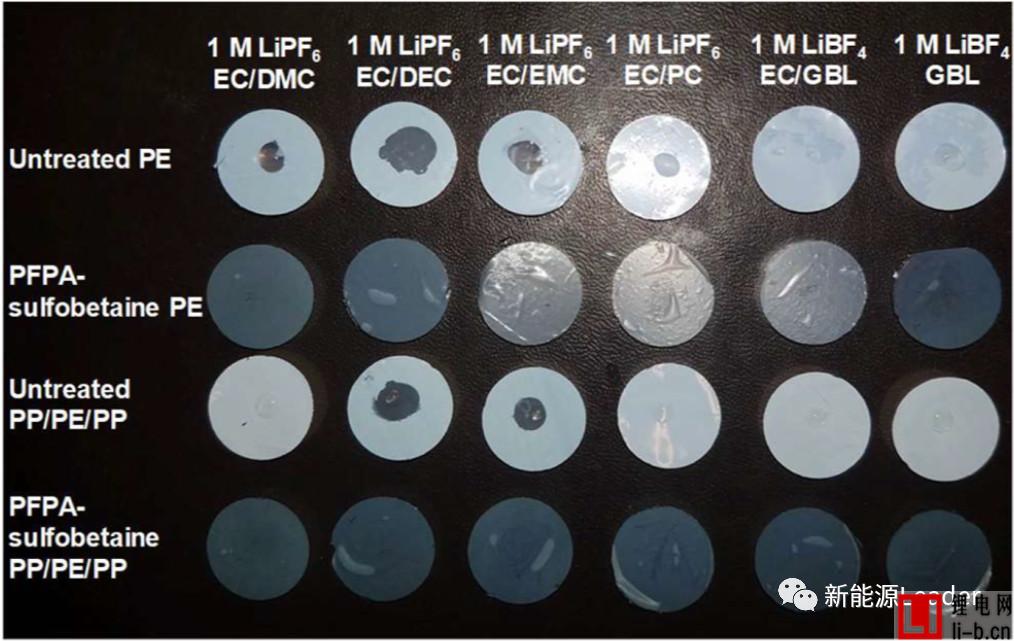
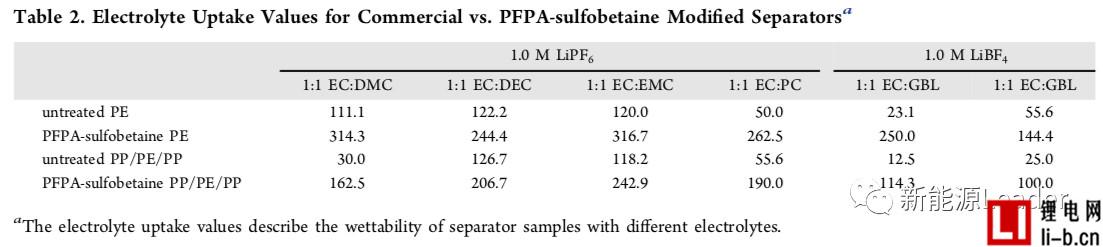
In order to improve the infiltration effect of the membrane and electrolyte, Ethan Rao et al. [3] of the University of California, USA, greatly improved the electrolyte by coating a layer of perfluorophenyl azide PFPA on the surface of a common polymer membrane. Wetting between the membrane and the membrane. The following figure shows the contrast of the treated separator and the common diaphragm in different electrolytes. From the figure, we can see that the ordinary single-layer PE separator has very poor wettability in the electrolyte, especially at the pole. In the latter electrolyte formulations, the electrolyte and the PE membrane are hardly wetted, but after the PFPA treatment, the PE membrane can be completely infiltrated in all the electrolyte formulations, and the effect is very obvious. The PP/PE/PP three-layer composite separator also exhibits a similar pattern, which greatly improves the infiltration effect of the electrolyte after surface treatment. The table below shows the electrolyte climb height data for the common diaphragm and the treated diaphragm. It can be seen that the surface treated membrane has a very significant advantage in the electrolyte climb height, which again demonstrates that the diaphragm surface modification treatment is improved. The electrolyte wettability has a very significant effect.
The good wettability of the separator can significantly improve the performance of the lithium-ion battery. The first is to reduce the internal resistance. As can be seen from the following figure A, the treated PE and PP are compared to the PE membrane without surface treatment. The internal resistance of the /PE/PP separator is significantly reduced. It can also be seen from the rate performance in Figure B that the surface treated PE separator has a significant advantage in the rate performance compared to the untreated PE separator, even if the PP/PE/PP three-layer composite membrane passes through the PFPA surface. The rate performance was also significantly improved after treatment, even surpassing the untreated PE single layer separator at some magnifications.
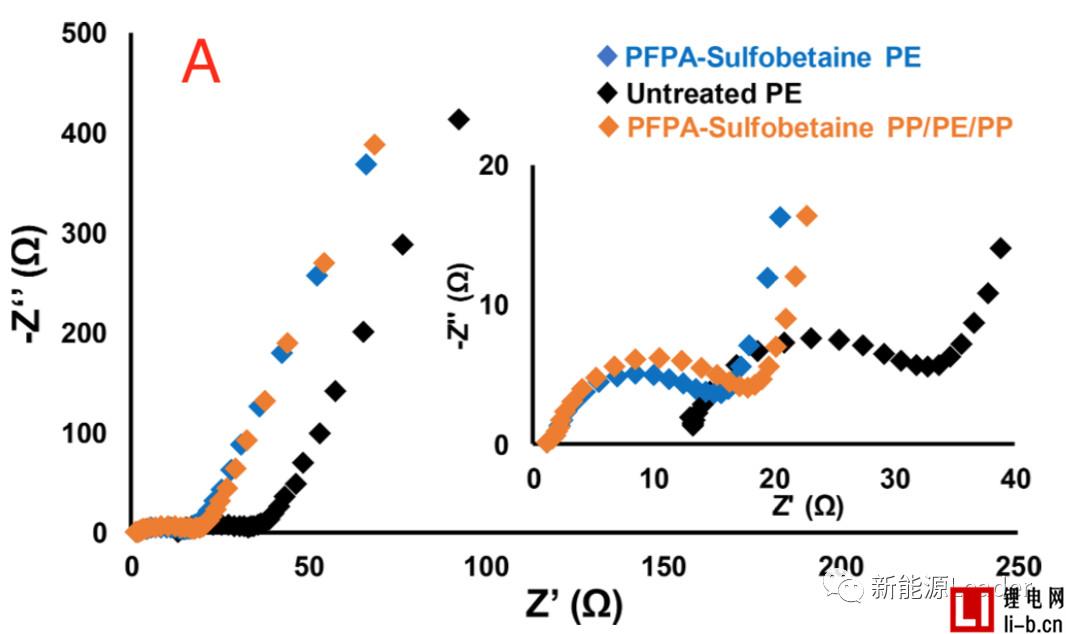
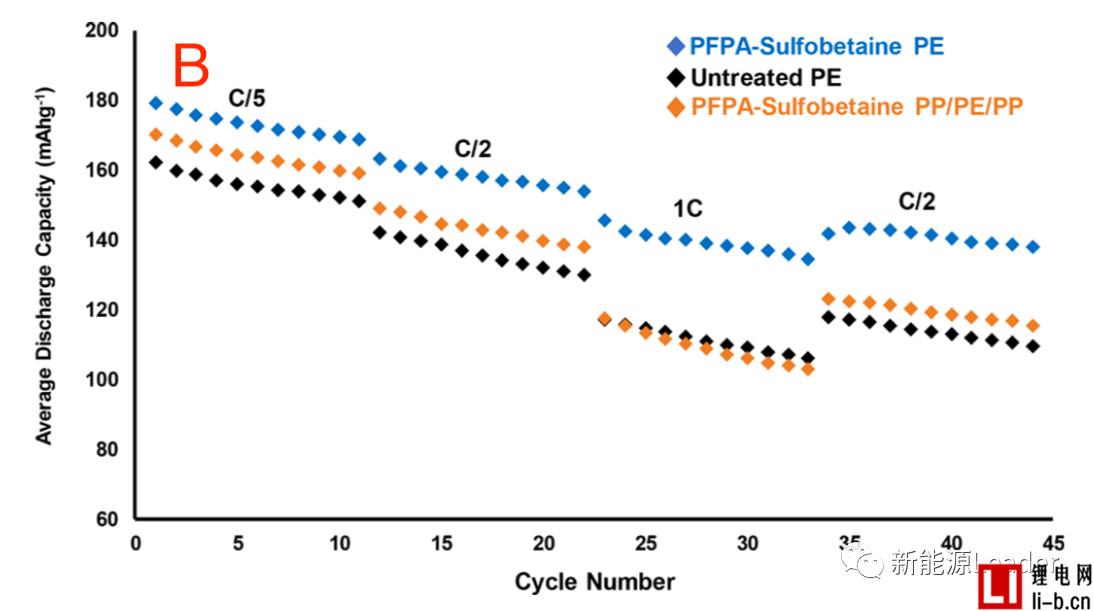
How to improve the infiltration effect of electrolyte on the cell is a key factor affecting the performance of lithium ion battery rate, cycle, etc. WJ Weydanz research shows that vacuum injection can significantly shorten the injection time and improve the quality of injection, while Thomas Knoche further analyzed The influence of the vacuum system on the liquid injection effect indicates that the higher the vacuum degree of the injection liquid and the more vacuuming before sealing, the better the infiltration effect of the final battery core. Ethan Rao's membrane surface modification greatly improves the wettability of common polymer membranes, significantly reducing the internal resistance of lithium-ion batteries and improving the rate performance of batteries. All of these tasks have only one purpose: to ensure that the electrolyte can "smooth" the infiltrated cells and improve the performance of the lithium-ion battery.


 Online map
Online map Collection Website
Collection Website











