新闻资讯NEWS |
详细信息
当前位置:
首页>
详细信息
Mechanism Analysis of High Temperature Decay of NCM622 Material
专栏:Industry Information
发布日期:2018-06-08
阅读量:2763
收藏:
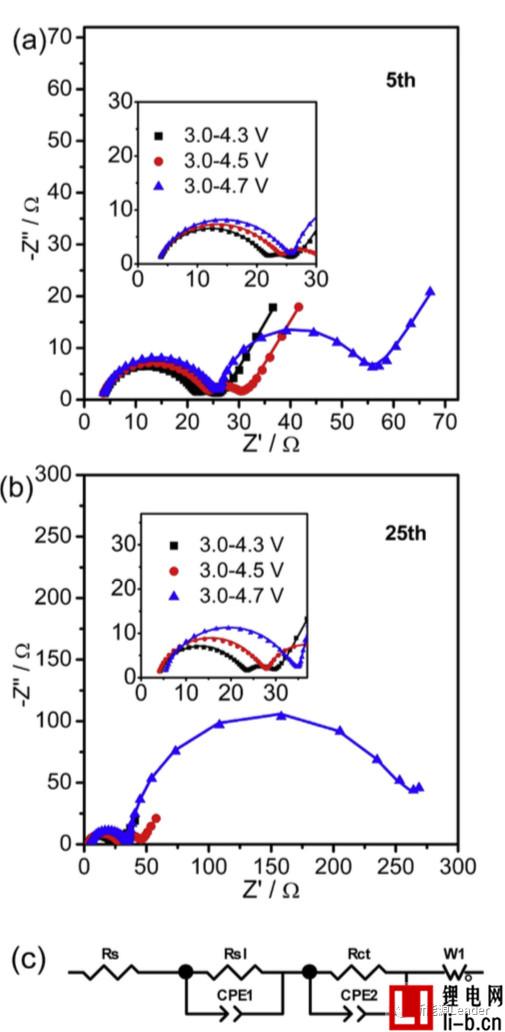
The following figure shows the EIS analysis results of NCM622 materials under different cut-off voltages and different cycle times.
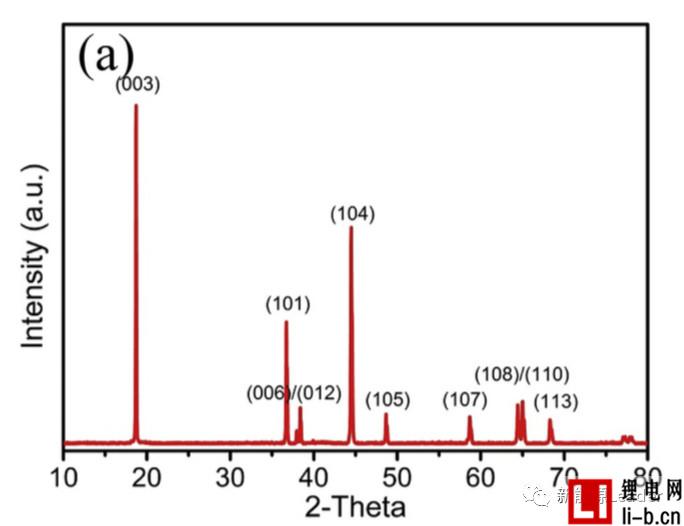
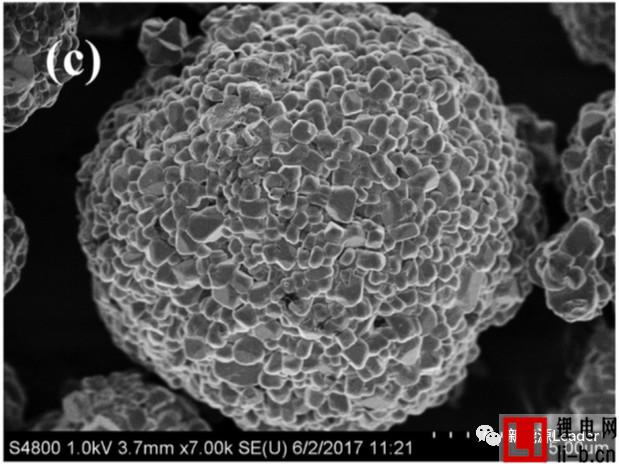
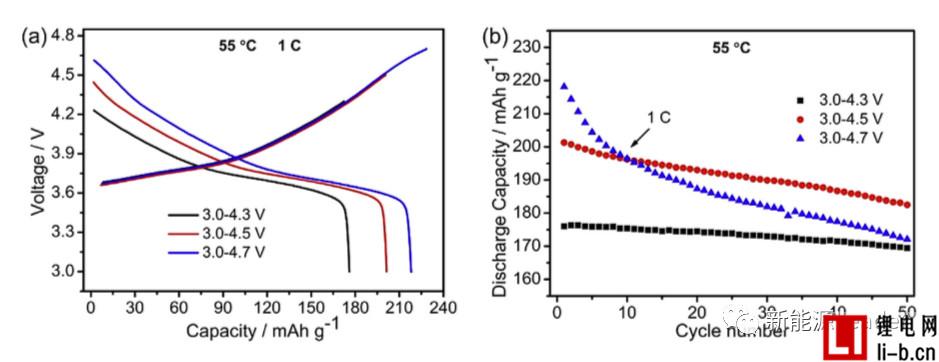
Siyang Liu first synthesized the NCM622 material by solid phase method. The XRD pattern showed that the synthesized NCM622 material had a well-developed layered a-NaFeO2 structure. Figure a below shows the first charge-discharge curve of Siyang Liu synthesized NCM622 material under different cut-off voltages. It can be seen from the figure that as the cut-off voltage is gradually increased to 4.3V, 4.5V and 4.7V, the material capacity is reached. 176, 201.3 and 218.1 mAh/g, although higher cut-off voltages can lead to higher capacity, but also cause a very rapid decline in the cycle performance of NCM622 materials. It can be seen from the following figure b that when the cutoff voltage is 4.3V, 4.5V and 4.7V, respectively, the capacity retention rate of the NCM622 material cycled at 55 °C for 50 times is 96.3%, 90.7% and 78.9%, respectively. The voltage has an important influence on the cycle performance of the NCM622 material.
The study of the decay mechanism of NCM622 materials at different cut-off voltages found that a higher cut-off voltage significantly increased the interfacial resistance of the NCM622 material. The following figure shows the EIS analysis results of NCM622 materials under different cut-off voltages and different cycle times. It can be seen that all the curves are composed of two arcs and a straight line, which indicates that there are two interfaces on the surface of the material: The surface of the NCM622 material is decomposed to form an interfacial film. Siyang Liu fits the EIS results using the equivalent circuit in Figure c below. Siyang Liu considers Rs1 as the impedance of the interface film and Rct as the charge exchange impedance. When the cutoff voltage is 4.3V, 4.5V and 4.7V, respectively, the Rs1 of the material is 17, 20 and 21.6W, respectively, and after 25 cycles, Rs1 is increased to 18.7, 23.4 and 28.2W, respectively, indicating a higher cutoff. The voltage causes the growth and reconstruction of the interfacial film of the NCM622 material, thereby increasing the interfacial resistance.
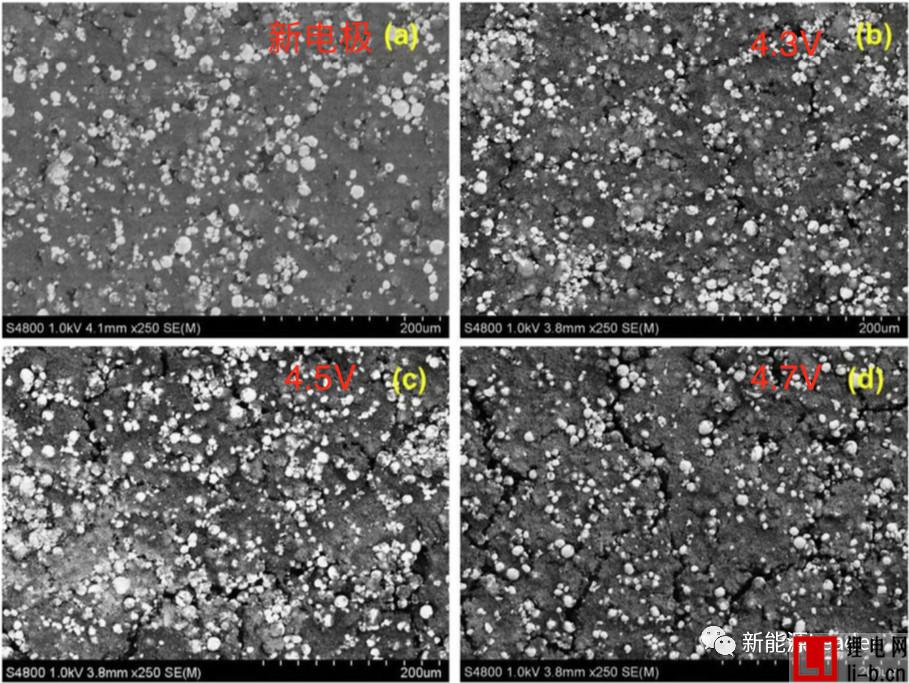
EIS analysis shows that the increase of the interface impedance of the material is closely related to the decline of the capacity of the material, but the mechanism of action is still unclear. The figure below shows the SEM picture of the new electrode and the electrode after cycling at different voltages. We can see that there is a significant increase in the number of cracks on the surface of the electrode after cycling, especially at the electrode surface after cycling at a higher cut-off voltage. The cracks become more serious. These cracks on the surface of the electrode may cause some of the active material to lose its connection with the Al foil and the conductive network, resulting in loss of the active material, resulting in partial capacity degradation.
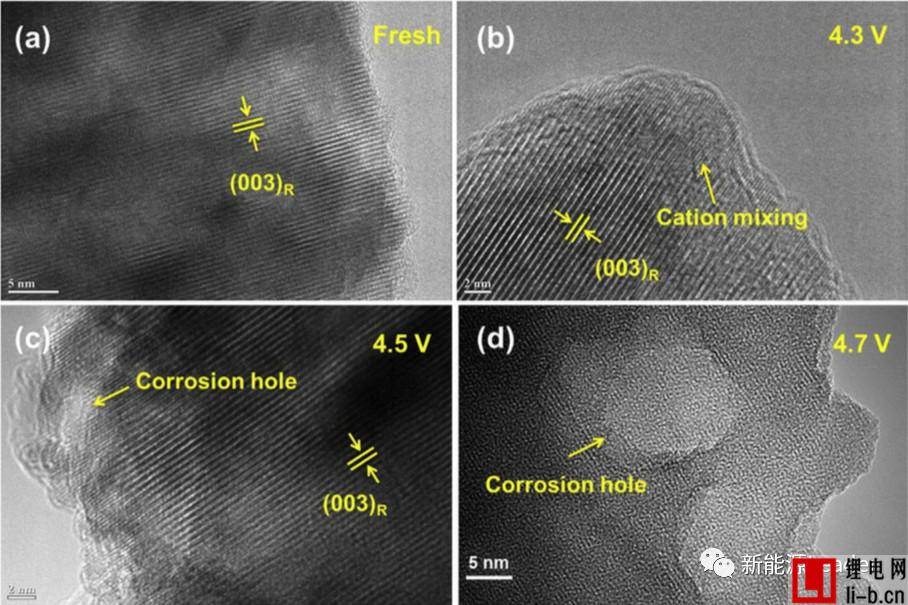
Generally, we believe that the side reaction mainly occurs at the electrode/electrolyte interface, so the electrode/electrolyte interface is more susceptible to erosion. Therefore, Siyang Liu used HRTEM to detect the surface layer of NCM622 after cycling at different voltages. Through high-resolution TEM images, we noticed that the new NCM622 has a well-developed crystal structure. After circulating 50 times at a cutoff voltage of 4.3V, the body of the NCM622 material still maintains a well-developed layered structure, but on the surface of the material. It can be observed that transition metal ions are mixed in some areas. When the cut-off voltage is increased to 4.5V and 4.7V, the crystal structure of the material decays more severely. It can be seen from the figure that excessive Li removal at high cut-off voltage causes metal cations to enter the Li layer, which blocks The diffusion channel of Li reduces the active site of Li, resulting in an increase in interface charge exchange impedance and a decrease in reversible capacity, which is consistent with previous EIS analysis results.
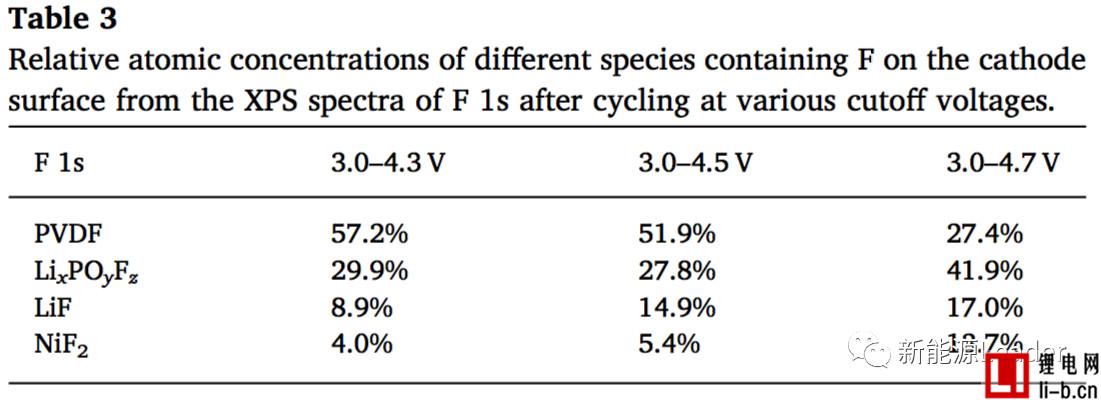
In view of the mechanism of the increase of the electrode/electrolyte interface resistance Rs1, Siyang Liu analyzed the surface of the NCM622 material by XPS and found that the electrolyte decomposition product showed a significant increase after the cycle. In particular, the LiF content of the electrode surface after LiF circulated at a cutoff voltage of 4.3V was 8.9%, but the LiF content of the electrode surface increased to 14.9% and 17% when the cutoff voltage was increased to 4.5V and 4.7V, while passing XPS. Analysis also found that the surface of the electrode showed a significant increase in the content of NiF2 after the cycle, which indicates that the decomposition process of the electrolyte on the surface of the NCM622 material is accompanied by the dissolution of the transition metal element. Siyang Liu believes that this is mainly due to the HF pair produced by the decomposition of LiPF6. The NCM622 material is corroded, causing the dissolution of transition metal elements.
The work of Siyang Liu shows that the circulation of NCM622 material at high temperature and high cut-off voltage will cause the increase of the transition metal element and Li in the material of the electrode surface, causing the decay of the surface crystal structure of NCM622 material, resulting in an increase in charge exchange resistance and The reversible capacity is reduced. Cycling at high temperature and high voltage also causes decomposition of LiPF6 on the electrolysis surface, resulting in an increase in the content of LiF and NiF2 on the surface of the NCM622 material, resulting in an increase in the electrode/electrolyte interface impedance of the NCM622 material.
|

 在线地图
在线地图 收藏本站
收藏本站