Lithium battery charging and discharging principle, lithium battery working principle
The working principle of lithium-ion battery refers to its charging and discharging principle. The more lithium ions return to the positive electrode, the higher the discharge capacity.
The working principle of lithium-ion battery refers to its charging and discharging principle. When the battery is charged, lithium ions are generated on the positive electrode of the battery, and the generated lithium ions move to the negative electrode through the electrolyte . The carbon as the negative electrode has a layered structure, and it has many micropores. The lithium ions reaching the negative electrode are embedded in the micropores of the carbon layer, and the more lithium ions are embedded, the higher the charging capacity.
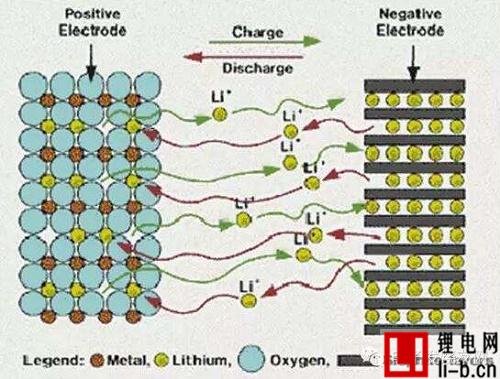
By the same token, when the battery is discharged (ie, the process we use the battery), the lithium ions embedded in the carbon layer of the negative electrode come out and move back to the positive electrode. The more lithium ions return to the positive electrode, the higher the discharge capacity. What we usually call battery capacity refers to the discharge capacity.
During charging and discharging of a lithium ion battery, lithium ions are in a state of motion from a positive electrode to a negative electrode to a positive electrode. If we compare the lithium-ion battery to a rocking chair, the two ends of the rocking chair are the two poles of the battery, and the lithium ion is like an excellent sportsman, running back and forth at both ends of the rocking chair. Therefore, the experts gave the lithium-ion battery a cute name rocking chair battery.


 在线地图
在线地图 收藏本站
收藏本站

